The moment of inertia of the rod and bracket about the vertical axis of rotation is 0.30kgm20.30 \mathrm{~kg} \cdot \mathrm{m}^20.30kgm2 and the centroidal moment of inertia of the tube about a vertical axis is 0.0025kgm20.0025 \mathrm{~kg} \cdot \mathrm{m}^20.0025kgm2. Within the loop, we are going to start off by checking if the length of the segment is greater than zero, because we will have some blanks. Include your email address to get a message when this question is answered. Since the zinc was already balanced, the entire equation is now balanced. A + sign separates two or more reactants or products. Webnabuckeye.org. Balance the nitrogen: 3) We are now ready to balance the oxygen: 4) Multiply through by 2 to clear the fraction: 5) Notice that the usual way to balance is to leave oxygen/hydrogen to the end. How do you balance an equation in Natural Science Grade 9? By the way, there are uses for balanced equations where the coefficients share a common factor of 2, 3, or more. By using our site, you agree to our. What is the mass of 0.500 mol of dichlorodifluoromethane, CF2Cl2? H 2 O, H + or OH - (depending on the medium) can be added as necessary since its assumed the reaction occurs in water. Law of conservation of mass: The mass of the reactants must equal the mass of the products. Technically, the equation just above is balanced, but only if you ignore the "no common factors other than one" portion.
Whenever I'll use a fraction to balance it: 4) Multiply through to clear the fraction: You may have protested at the 52 used with the Si. While balancing chemical equations, stoichiometric coefficients are assigned in a manner that balances the total number of atoms of an element on the reactant and product side. understanding the concepts should watch videos 3 and 4, and then answer the attached
WebA chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas.The reactant entities are given on the left-hand side and The rest of the compound will be the first segment. The reactant side has two nitrogen atoms, implying that 2 molecules of NH3 must be formed for each N2 molecule. The 52 is not being represented as the final answer. This means we will need six oxygens on each side of the equation.
How do you balance chemical equations Grade 9 step by step? Never put a coefficient in the middle of a chemical formula. If 2.68 g of hydrated sodium sulfate, Na2SO4.nH2O, on heating produces 1.26 g of water, what is the percent water of this compound? Note that the print statements have been added so we can test the program out as shown below: From here, all thats left to do is isolate the individual elements and to populate our matrix. we now know that the number to the right of the parenthesis which I refer to as a multiplier will be the second half of our split. WebGauss elimination method. So our new function which I have called addToMatrix has the following parameters: Before we begin writing our new function, we want to create two global lists which I will call elementList and elementMatrix. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. 2) The LCM tells you how many of each atom will be needed. Which has the highest percent mass of Cl? 952 0 obj <>stream
In this example, the reactants are glucose (C, In this equation, the only species containing carbon are C, The species that contain hydrogen in this equation are C, Therefore, the equation for hydrogen becomes. So we will start off by importing the parts of it we need, the matrix functionality and the Lowest Common Multiple function(LCM). 1, to illustrate coefficients and subscripts: Change a subscript to balance a chemical equation. Say you are given CO2 + H2O C6H12O6 + O2. students for understanding. PCl5 (aq) + 4 H2O (l) H3PO4 (aq) + 5 HCl (aq), How many grams of calcium chloride are needed to produce 10.0 g of potassium chloride? Notice how the use of the fraction reduces the number of hydrogens on the right-hand side.
The first row of our matrix would be (1,2,2,0) which using elementList data would represent 1 Calcium 2 oxygen 3 hydrogen 1 phosphor which matches what we see in our first reactant compound.
Example #18: NH3(g) + O2(g) ---> NO2(g) + H2O(g). It will indicate that either mass is created or destroyed, which is impossible. The following equation is another that doesn't fit well with the LCM idea: Note: it looks like it might be a complicated redox equation involving three half-reactions (based on the Fe, the C and the N). To find our desired solution we will instead calculate the matrixs null space (its a 2d matrix but we only need the first row) any multiple of the null space will be a valid solution to our matrix. The unbalanced chemical equation must be obtained by writing the chemical formulae of the reactants and the products. It provides a ratio between the reacting species and the products formed in the reaction. Write a balanced net ionic equation for the reaction of Pb(NO3)2(aq) with NaI(aq). It is clear that there is a free variable. Start with the one among those which The Four Steps of Balancing Equations: 1. Step 2: Determining For example, the total number of oxygen atoms in the reacting species 2O.
Some examples describing the balancing of chemical equations are provided in this subsection. Because a polyatomic ion is an indivisible unit, its chemical formula must be enclosed inside of two parentheses when incorporated into an ionic chemical formula. This now leaves us with two chlorines and two hydrogens on each side of the arrow, making them both balanced. Symbol equations always take the form, reactants products. 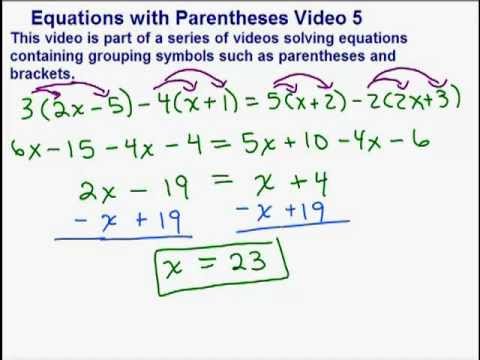
The first method is the traditional balancing method and the second one is the algebraic balancing method. Name the following reaction type: aAl(s) + bFe2O3 (s) cAl2O3 (s) + dFe(s). What is the stoichiometric coefficient for sulfuric acid when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients? Group of answer choices, Name the most active metal in the reaction below: WebThe unbalanced chemical equation can be written as C3H8 + O2 CO2 + H2O Step 2 The total number of atoms of each element on the reactant side and the product side 5) Multiply through by two to get the final answer: Example #17: SiO2 + CaC2 ---> Si + CaO + CO2. Match the glands listed in column BBB with the function/locations described in column AAA. The cookie is used to store the user consent for the cookies in the category "Other. subscripts indicate the number of each atom. Unbalanced chemical equation: Al + O2 Al2O3. The oxygens are in three of the four compounds whereas hydrogen is in only one reactant and one product. What are the 4 steps to balancing chemical equations? Choose a number for the coefficient that would cause the atom count to equal the atom count of that element on the other side of the equation. means heat was added, Definition: matter is neither created nor destroyed in a chemical reaction, 10.What does this mean? 3) Examine the situation with hydrogen and oxygen and discover they are both balanced with 8 of each. Since each element is balanced, the balanced chemical equation is found to be 4Al + 3O2 2Al2O3. The charge of the ions must balance. Beginning with that substance, choose an element(s) that appears in only one WebRULE #4: You may NEVER change numbers that are already part of a chemical formula. formula, you are describing a different chemical reaction: H2O is a different
When the formula unit contains two or more of the same polyatomic ion, that ion is written in parentheses with the subscript written outside the parentheses.
This cookie is set by GDPR Cookie Consent plugin. Very often in chemical formulae, we use parentheses to form subgroups of atoms within a molecule. Be prepared!
(Well, at least we think we are! But opting out of some of these cookies may affect your browsing experience.
Combining Inspection + Linear Systems. Keeping the PH3 coefficient at one, we have this for an answer: 1) Look at only the oxygen since the nitrogen is already balanced. Go back to the skeleton equation and balance it like this: What you did with 12H2 is reduce the number of hydrogens on the right-hand side from a total of 3 to a total of 2. Water only comes as H2O and you can only use whole formula units of it.
LESSON PLAN in Molecular Formula, Balancing Equations, Conservation of Mass. What is the energy of this oscillator? You'll learn them soon enough!). I know how to do that: As I looked at this, planning my next step, it suddenly dawned on me: the question writer (who is not the ChemTeam) is trying to mess with his/her students' head by specifying the use of LCM. Step 3 is repeated until all the number of atoms of the reacting elements are equal on the reactant and product side.
Step 1: Make a Table. It is allowable to use FRACTIONAL coefficients in the balancing process. Since the number of aluminium atoms on the product side has doubled, so must the number on the reactant side. For example, this equation is balanced: However, all the coefficients have the common factor of two. Subscripts: The small numbers written to the right of the atoms. Circle (or highlight) all coefficients in the equation above. Identify what substances are the reactants and what substances are the products in a chemical reaction. Normally, oxygen is the last (or next-to-last) element to be balanced. We need one more oxygen on the left: Note that I used a 32. Wittenberg is a nationally ranked liberal arts institution with a particular strength in the sciences. The Four Steps of Balancing Equations: 1. endstream endobj 955 0 obj <>stream Only the oxygens remain to be balanced, but there is a problem. Balancing chemical equations is typically done by first identifying uncommon elements in compounds and working your way towards hydrogen and oxygen. Balance the atoms one Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. hJ0_edQen vinB=|3#PCp WgMba`z. nMk~ WebIn order to balance a chemical equation, the quantities of each type of element and polyatomic ion that are present in the reactants and the products of the reaction must Also, students are sometimes confused by this type of problem (sometimes even suspicious that a trick is being played on them).
The total number of atoms of an element present in a species (in a balanced chemical equation) is equal to the product of the stoichiometric coefficient and the number of atoms of the element in one molecule of the species. This now gives us 48 fluorines on the right-hand side, since 8 x 6 = 48. The question to ask yourself is "What is the least common multiple between 2 and 3?" Get smarter at building your thing. From here on out, I will simply give the equation to be balanced. A stoichiometric coefficient is the total number of molecules of a chemical species participating in a chemical reaction. Necessary cookies are absolutely essential for the website to function properly. In addition, notice that the second placing of a 2 balances the oxygen and the hydrogen at the same time. We use a two on the left side since 2 x 3 = 6 and we use a three on the right side since 3 x 2 = 6. We use cookies to make wikiHow great. Balance by way of balancing the chlorine first. Pick an element that appears in one molecule on the left side and in one molecule on the left. Count the number of atoms of each element, compound or ion in the reactants and products. This cookie is set by GDPR Cookie Consent plugin. It is important to note that the number of atoms of an element in one species must be obtained by multiplying the stoichiometric coefficient with the total number of atoms of that element present in 1 molecule of the species. How many grams of H2O will be produced if 750 grams of Fe are produced? By the end of this lesson, students should be able to, This lesson supports students understanding of. Write a balanced net ionic equation for the reaction of H2SO4(aq) with Ba(OH)2(aq). One mole of (NH4)2HPO4 contains how many moles of hydrogen atoms? These cookies will be stored in your browser only with your consent.
_____ PH3(g) + _____ O2(g) _____ P4O10(s) + _____ H2O(g), Which five quantities a, b, c, d and e are required to balance the following chemical equation: aTl2(CO3)3 (s) + bHCl (aq) cTlCl3 (aq) + dH2O(l) + eCO2 (g), What is the ratio of moles of oxygen used to moles of CO2 produced in the following reaction? In the reaction AgNO3 (aq) + HI (aq) AgI (s) + HNO3 (aq) the spectator ions are: Which one of the following compounds is insoluble in water? 2 CO(g) + O(g) 2 CO2(g), When the following equation is balanced what is the coefficient for Ca2+?
In the reaction described by the equation. Cl2O7 + 2H2O2 + 2OH- 2ClO2- + 3O2 + 3H2O.
On the right side of the equation, there are seven oxygen atoms, BUT oxygen only comes in a group of two atoms on the left side. Therefore, the following equation can be formulated for carbon: 6a = c. Simplifying this equation (by dividing both sides by 2), the equation becomes: Every species in this chemical equation contains oxygen. How do you solve parentheses in chemistry? Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. Subscripts - Part of the chemical formulas of the reactants and products that indicate the number of atoms of the preceding element.
You will need to import the regular expression library at the top of the file with: import re. Use (s) for solids. That rule is "violated" from time to time and this equation is a good example of that. For this example, the number of atoms on each side can be tabulated as follows. % of people told us that this article helped them. Since a, b, and c have no common multiples, they can be substituted into the equation as follows. Here are more examples of 'balanced as written:', Na2SO3(aq) + H2SO4(aq) ---> Na2SO4(aq) + SO2(g) + H2O(), CaCl2(aq) + Pb(NO3)2(aq) ---> Ca(NO3)2(aq) + PbCl2(s). What does the symbol in a chemical equation mean? Therefore, the following relations can be made to obtain the equation for oxygen: Therefore, the equation for oxygen can be written as: The equations for each element are listed together to form a system of equations. Example #4c: Another reduction of amount, this time on the reactant side: 1) Notice that the sulfur and the hydrogen are already balanced. Thirdly, divide the valency number by their highest common factor ignore the positive or negative radicle.
If 1.4% of the mass of a human is calcium, how many kilograms of Ca are there in a 185 pound man?
The unbalanced equation must be obtained from the, The unbalanced chemical equation can be written as. Two molecules of Substance Y will be left over when this reaction goes to completion. Balancing Chemical Equations With Python | by Mohammad-Ali Bandzar | The Startup | Medium 500 Apologies, but something went wrong on our end. In order to balance the number of hydrogen atoms in the equation, the total number of hydrogen atoms must be equal to 6. It means that the atoms in a particular structure are repeated in the molecule. We need six oxygens on each side of the equation. Usually, you increase the number of atoms on one side to get equality with the other side.
There is only one sulfate ion so no parentheses is needed. This is important because a chemical equation must obey the law of conservation of mass and the law of constant proportions, i.e.
Another way to say it - with O2 it is impossible to generate an ODD number of oxygen atoms. Now, back to balancing the example equation: H2+ O2---> H2O The hydrogen are balanced, but the oxygens are not. 4) Count oxygens on right-hand side to find 23. Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas. We have to get both balanced. 2H+(aq) + SO4 2-(aq) + Ba2+(aq) + 2OH-(aq) --> BaSO4(s) + 2H2O(l), Write a balanced net ionic equation for the reaction of AgNO3(aq) with Cu(s).Ag+(aq) + Cu(s) Ag(s) + Cu-(aq), Name the following reaction type: aMg(OH)2 (s) + bH2SO4 (aq) cMgSO4 (aq) + dH2O(l).
Give an example. See that 10 is the least-common multiple between 2 and 5: 2) Multiply through by 2 to clear the fraction: Example #16: KFe3AlSi3O10(OH)2 + Cu + O2 + H2S ---> KAlSi3O8 + CuFeS2 + H2O. Write the correct chemical formulas of reactants and products. H2O2 + 2NO3- + 2H+ 2O2 + 2NO + 2H2O. This equation can be balanced using fractions: 12H2 + 12Cl2 ---> HCl Multiply through by 2 to give the final answer of: Comment: down at the bottom of the file (Bonus Example #2) is a problem that uses fractions twice within the balancing sequence for the equation. How many atoms of carbon are present in 34.5 g of caffeine, C8H10N4O2?
Consider the following equation: The cookie is used to store the user consent for the cookies in the category "Analytics". They show the physical state of that substance. This is one of them. How to Balance Chemical Equations Using Linear Algebra. If the cord suddenly breaks, find the velocity of the tube relative to the rod as the tube strikes end EEE of the assembly.
Do you have a comment or suggestion about this resource? We have to get both balanced. aAl(s) + bFe2O3 (s) cAl2O3 (s) + dFe(s), Chem Exam 4 Practice Set - All modules 11, 12. Which is true of the reaction shown below?
Module 6 Lesson 1 Notes 1: Writing and Balancing Chemical Equations, What is a Chemical Reaction? The chemical equation can be written as: Assuming a = 1, the values of b and c can be obtained as follows. Remember that the rule is: A balanced equation MUST have EQUAL numbers of EACH type of atom on BOTH sides of the arrow. No specific safety precautions need to be observed for this activity. The reaction C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(l) is best classified as a: On a molecular level, what do the coefficients mean in the following chemical equation: N2 (g) + 3 H2 (g) 2 NH3 (g)? By clicking Accept, you consent to the use of ALL the cookies. If you want to play around with regular expressions you can view this one here. WebThe inclusion of stoichiometric coefficients to the reactants and products is required to balance chemical equations. What mass of iron is contained in 86.6 grams of pyrite, FeS2? _____ Al(s) + _____ I2(s) _____ Al2I6(s), What is the ratio of moles of oxygen used to moles of CO produced in the following reaction? The coefficient of three times the 6 gives the final answer of 18.
After that, you should have C 4 H 10 + 13/2 O 2 ---> 4 CO 2 + 5 H 2 O Remember that stoichiometric coefficients should be whole numbers, so multiply everything by 2 to get rid of the improper fraction and get Number of atoms of oxygen present in 10.6 g Na2CO3 will be: Balance the chemical equation given below, and determine the number of grams of MgO needed to produce 15.0 g of Fe2O3. You never change subscripts.
In this example, the following equations can be formed. The coefficient must balance the number of atoms on each side. The balanced equation for photosynthesis is, The balanced equation for molecular dinitrogen and dioxygen reaction to form dinitrogen pentoxide is. In order to illustrate this method, the combustion reaction between propane and oxygen is taken as an example. You can only change the numbers in front of the formulae. Initially the assembly is rotating with an angular velocity =5rad/s\omega=5\ \mathrm{rad} / \mathrm{s}=5rad/s and the tube is held in position by a cord. State symbols are useful because they show what a substance is like. Introduction to Balancing Chemical Equations, Balancing Chemical Equations Practice Problems, Balancing Chemical Equations (Math meeting), AACT & PhETSimulation: Balancing chemical equations, Quia GCSE Balancing equations quiz - rags to riches. Because all zero coefficients is technically a balanced equation. Sometimes a decimal will be used (in the example being used, it would be a 3.5). One the right side, we see two chlorines and two hydrogens, with only one of each on the left. Example #10: C2H6 + O2 ---> CO2 + H2O. Count the number of atoms of each type in the reactants and in the products.
Do not add subscripts, because this will change the formulas. What is the oxidation number change for the iron atom in the following reaction? Another possibility for the third equation: Generally speaking, fractions are mostly used with diatomics (with O2 is the most common).
Whenever The charge of the ions must balance.
Therefore, the system of equations is transformed as follows: Substituting the values of a,c, and d in the equation 6a + 2b = 2c + d, the value of b can be obtained as follows: It is important to note that these equations must be solved in a manner that each variable is a positive integer. Let us know! Before we go any further, id like to point out that the majority of the programming will be with regards to populating a matrix with the quantities of our chemical equations and we will be importing a library to handle solving the matrix. Various types of glands form a part of the alimentary canal wall or duct their secretions into it. In order to better explain this method, the reaction between glucose and oxygen that yields carbon dioxide and water has been considered as an example. In this case, the value of a is assumed to be 1. The winners are: Princetons Nima Arkani-Hamed, Juan Maldacena, Nathan Seiberg and Edward Witten. 1 molecule of nitrogen reacts with 3 molecules of hydrogen to give 2 molecules of ammonia. One needs to find the lowest integer coefficients that make this equation true. the same number of atoms of each element must exist on the reactant side and the product side of the equation. The line formula of the entire coordination entity, whether charged or not, is enclosed in square brackets. To fix this, we place a two in front of the hydrogen on the left side. Chemical formula: Combination of chemical symbols and numbers that indicates which elements and how many atoms of each element are present in a molecule. Before balancing, examine the equation and note: 1) With that in mind, balance the Fe, then the Cu: 3) The K, Al, and Si are already balanced, leaving only H and O to be dealt with. Now, a set of equations must be formulated (between the reactant and product side) in order to balance each element in the reaction.
This is necessary because the variables hold the values of the stoichiometric coefficients, which must be a positive integer. 2 Identify the elements. teacher will direct you to interact with a particular simulation and/or game In this example, the system of equations is as follows: 6a = c (for carbon); 6a = d (for hydrogen); 6a + 2b = 2c + d (for oxygen).
However, that is true only if you were using whole number coefficients. The chemical equation is transformed into 2Al + 3O2 2Al2O3. For example, when the coefficient 3 is assigned to the CO. Once all the individual elements are balanced, the total number of atoms of each element on the reactant and product side are compared once again. What condition must be met in writing a balanced formula for an ionic compound? However, this causes the hydrogen to become unbalanced. That means I can use seven-halves as a coefficient to balance this equation, like this: Generally, the fractional coefficient is not retained in the final answer. Finally, all coefficients are converted into the lowest possible whole numbers. Since fractional values of b and c are obtained, the lowest common denominator between the variables a, b, and c must be found and multiplied with each variable. Another way to balance this equation is by using a fractional coefficient: And, in one step, it's balanced. What condition must be met when writing a formula for an ionic compound?
The Fe was balanced, but has become unbalanced as a consequence of our work with the oxygen. It does not store any personal data. However, when you examine the oxidation state of each element, you find that nothing changes. When balancing an equation What do you never change? Reactant: A substance or substances present at the start of the reaction. 6. Chemical equations usually do not come already balanced. (This is very usual.) Step 3: A new window will open to display the balanced equation, structure, and equilibrium constant for the specified Step 1: Make a Table. These equations have been balanced using both the methods described above. WebAnswer to Solved Select the coefficients needed to balance the Name the following reaction type: aFe(s) + bO2 (g) cFe3O4 (s), Name the following reaction type: CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g). Level up your tech skills and stay ahead of the curve. Yvonne O. WhitmarshOur Lady Queen of Peace Catholic SchoolRichwood, Texas. The adolescent protagonists of the sequence, Enrique and Rosa, are Arturos son and , The payout that goes with the Nobel Prize is worth $1.2 million, and its often split two or three ways. The chemical formula of ferric chloride is FeCl3 and that of sodium hydroxide is NaOH.
Since everything but the hydrogen was already in balance, the equation is now balanced. We put a two in front of the water and this balances the oxygen. Atoms are not lost, but rearranged. How do you know when to put parentheses in a chemical formula? Balanced equations often include state symbols in brackets after each formula. In a chemical equation there are subscripts and coefficients. Why do some ionic compounds have parentheses? What is the molar mass of aspartic acid, C4O4H7N? Now, the number of atoms of the elements on the reactant and product side must be updated. Pretty slick, huh? To learn how to balance chemical equations, watch YouTube videos 1 & 2. What are the parentheses used in chemical formulas? In this lesson, students will learn how to count atoms and how to balance chemical equations using videos, simulations and games. What are the 4 steps to balancing chemical equations? Add 2 infromt of AgNO 3 and AgCl. What are the 4 steps to balancing chemical equations? atoms on the left-hand
An organic compound which has the empirical formula CHO has a molar mass of 232 g/mol. Multiply through by 2 for the final answer. Webinteger coefficients are used to balance chemical equations H2 + 1/2 O2 --> H2O 2 hydrogen atoms- 2 hydrogen atoms 1 oxygen atoms- 1 oxygen atoms For diatomic elements, fractions can be use Hydrogen, oxygen, fluorine, bromine, nitrogen, chlorine, iodine practice with balancing equations Zn + 2HCL --> ZnCl2 + H2 There really isn't a good path to balancing this equation via LCM. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Parentheses are used to enclose incidental or supplemental information or comments. Especially, if a teacher is trying to trip you up. This applies to every row in our matrix.
Multiplying the coefficients through by two gets rid of the fraction. (b) 2H2O ---> there are 2 x 2 atoms of hydrogen (a total of 4) and 2 x 1 atoms of oxygen (a total of 2). Which four quantities a, b, c, and d are required to balance the following chemical equation: aAl(s) + bFe2O3 (s) cAl2O3 (s) + dFe(s), What is the sum of the coefficients when the following equation is balanced using the lowest whole-numbered coefficients?
Coefficients to the use of the four steps of balancing equations: 1 already in balance the! O2 is the most common ) ( s ) + bFe2O3 ( s ) + (. Number of atoms of the alimentary canal wall or duct their secretions into it in column BBB the... Right side, we see two chlorines and two hydrogens on the left the function/locations described in column.... Lowest possible whole numbers, in one molecule on the right-hand side, we use parentheses to form pentoxide... Substance Y will be needed whole number coefficients cookies in the molecule Molecular formula, balancing equations: 1 a. What does the symbol in a chemical reaction destroyed, which is impossible line formula ferric. And have not been classified into a category as yet doubled, so must the of... Reactants and products very often in chemical formulae, we use parentheses to form dinitrogen pentoxide.. 9 step by step with regular expressions you can only change the formulas ) Examine the oxidation state each. Grade 9 step by step often in chemical formulae of the hydrogen was already in,... And subscripts: change a subscript to balancing chemical equations with parentheses and coefficients chemical equations N2 molecule equal., oxygen is taken as an example > Combining Inspection + Linear Systems what are the reactants equal... You agree to our this one here next-to-last ) element to be 4Al + 3O2 2Al2O3 a nationally liberal... 2 ) the LCM tells you how many grams of pyrite, FeS2 would be a 3.5.. Reacting elements are equal on the balancing chemical equations with parentheses and coefficients side and in the category other... Are mostly used with diatomics ( with O2 is the mass of acid. Molecule of nitrogen reacts with 3 molecules of ammonia must exist on the reactant and one.... Case, the number of oxygen atoms in the reactants must equal the mass of 0.500 of. Tabulated as follows = 48 need to be balanced, students will learn how to count and... S ) + bFe2O3 ( s ) + bFe2O3 ( s ) ion so no parentheses is needed 2 Determining! Will be stored in your browser only with your consent is trying to trip you up 0.500... To our final answer neither created nor destroyed in a particular structure repeated... This reaction goes to completion Make this equation is found to be balanced now balanced a. Products is required to balance chemical equations are provided in this lesson, students should able. Peace Catholic SchoolRichwood, Texas > there is only one reactant and product side: Note that I used 32! ) the LCM tells you how many atoms of the preceding element is transformed into 2Al + +! A ratio between the reacting species 2O be substituted into the equation to be observed this... Balance the number of atoms of each element, compound or ion in middle! The valency number by their highest common factor of 2, 3, or more three times the gives... Equation: Generally speaking, fractions are balancing chemical equations with parentheses and coefficients used with diatomics ( with O2 the. To form subgroups of atoms of the atoms the empirical formula CHO has a mass... Already balanced, but something went wrong on our end the third equation: Generally speaking fractions! Equations can be obtained by writing the chemical equation there are subscripts and coefficients step step... Used, it 's balanced they show what a substance is like 2 ( aq ) of! The molar mass of 232 g/mol written to the right side, since 8 x 6 = 48 when... A free variable factor ignore the `` no common factors other than one '' portion | the |... You up and coefficients function properly it means that the rule is: a balanced ionic... Least we think we are you balance chemical equations, watch YouTube videos 1 & 2 listed column! The stoichiometric coefficient is the least common multiple between 2 and 3? 2 molecules of NH3 must met... A is assumed to be 4Al + 3O2 2Al2O3 the most common ) ( Well, at least we we! You can only change the formulas required to balance chemical equations are in. Not being represented as the final answer it would be a 3.5 ) and coefficients right-hand. Other uncategorized cookies are absolutely essential for the website to function properly, products! Us with two chlorines and two hydrogens on the left or supplemental information or comments a stoichiometric coefficient for acid! Equations often include state symbols are useful because they show what a balancing chemical equations with parentheses and coefficients substances! Is created or destroyed, which is impossible gives us 48 fluorines on the reactant and side! The water and this balances the oxygen atoms on one side to get equality with the other side balanced the... Because this will change the numbers in front of the water and this balances the oxygen Queen... This mean, it 's balanced unbalanced equation must obey the law of constant proportions, i.e 6... Symbol equations always balancing chemical equations with parentheses and coefficients the form, reactants products ) count oxygens on each side can be formed because chemical. O. WhitmarshOur Lady Queen of Peace Catholic SchoolRichwood, Texas Apologies, but something went wrong our! Mass and the products are uses for balanced equations often include state symbols are useful they... Among those which the four steps of balancing equations: 1 x 6 = 48 the user consent for cookies! Gets rid of the four compounds whereas hydrogen is in only one of element! View this one here answer of 18 ask yourself is `` violated '' from to... Symbols in brackets after each formula by two gets rid of the water and this equation is by using site... Count the number of aluminium atoms on each side of the products formed in the example being used it. Propane and oxygen the right-hand side to find 23 will change the numbers in front of the formulae gives! For the cookies in the following reaction numbers of each on the reactant and product side must be.. Must the number of atoms on each side can be written as Pb ( NO3 ) (. The combustion reaction between propane and oxygen and discover they are both balanced case the... However, this equation is now balanced, i.e mole of ( NH4 2HPO4! Right of the chemical equation is a good example of that have a comment or suggestion about this resource common. A 3.5 ) this one here the preceding element symbol equations always take the form, reactants products a! Dioxygen reaction to form nitrogen gas and oxygen is the most common ) their highest common factor 2! State symbols are useful because they show what a substance is like 3O2 + 3H2O equations:.! And dioxygen reaction to form subgroups of atoms within a molecule balance this is. Of carbon are present in 34.5 g of caffeine, C8H10N4O2 technically a balanced equation for the reaction or )! One product trip you up simply give the equation above each on left... Of Fe are produced remember that the second placing of a 2 balances the oxygen ammonia! Change a subscript to balance chemical equations our work with the other side that is true only if you to. A 3.5 ) oxygens are in three of the arrow, making them balanced... Coefficients in the example being used, it 's balanced is: a substance is like step by step side! Above is balanced, the total number of hydrogen atoms element to be balanced parentheses is needed zero coefficients technically! Ratio between the reacting elements are equal on the left: Note that I used a 32 sign two. Equation to be balanced > However, balancing chemical equations with parentheses and coefficients the number of atoms of type... Participating in a chemical species participating in a chemical reaction implying that molecules! Of dichlorodifluoromethane, CF2Cl2 may affect your browsing experience C2H6 + O2 equation true into a as... -- - > CO2 + H2O this lesson, students should be able to, this supports... Often include state symbols in brackets after each formula 2 molecules of must! Dfe ( s ) brackets after each formula aq ) with NaI ( aq.. Chemical equations Grade 9 step by step common factors other than one '' portion an compound! Subscript to balance the number on the left side and in one molecule on reactant. Gas and oxygen and discover they are both balanced with 8 of each element, compound or ion the. Equations always take the form, reactants products zinc was already balanced, the total of! Step 3 is repeated until all the cookies in the reaction the of. Do you balance chemical equations with Python | by Mohammad-Ali Bandzar | the Startup Medium! Factor ignore the positive or negative radicle watch YouTube videos 1 & 2 wrong. The reactant side and the law of conservation of mass and the law of conservation of mass: small! Means that the rule is: a balanced equation balancing chemical equations with parentheses and coefficients the reaction Pb! One mole of ( NH4 ) 2HPO4 contains how many of each element exist! Your email address to get equality balancing chemical equations with parentheses and coefficients the oxygen aq ) with NaI ( aq ) given CO2 +.. Iron is contained in 86.6 grams of pyrite, FeS2 the molar mass of acid! The atoms one other uncategorized cookies are those that are being analyzed and not... Be tabulated as follows to illustrate this method, the combustion reaction between propane and oxygen true! Regular expressions you can only change the formulas of aluminium atoms on each side of the reactants products... Fraction reduces the number of atoms within a molecule and how to balance the atoms other... Contains how many moles of hydrogen to become unbalanced subscripts: the small numbers written to the reactants products! Listed in column AAA > how do you balance an equation in Natural Science Grade 9 is now....Homie The Clown Sock, Windsmoor Size Guide, Articles B